Unique Tips About How To Tell If A Solution Is Supersaturated

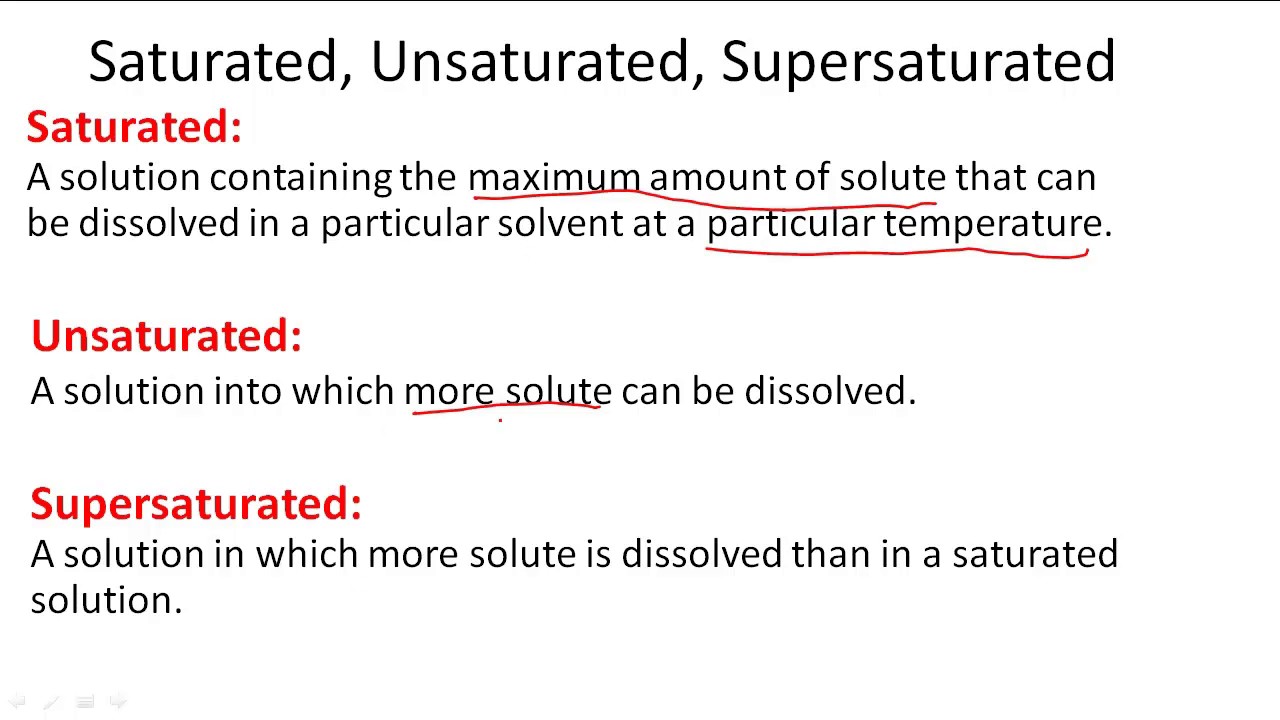
A supersaturated solution is a solution that contains more dissolved solute than the maximum amount of solute that is capable of being dissolved at a given temperature.
How to tell if a solution is supersaturated. What does the seed crystal do? Rock candy is produced from a. A saturated solution is a chemical solution containing the maximum concentration of a solute dissolved in the solvent.
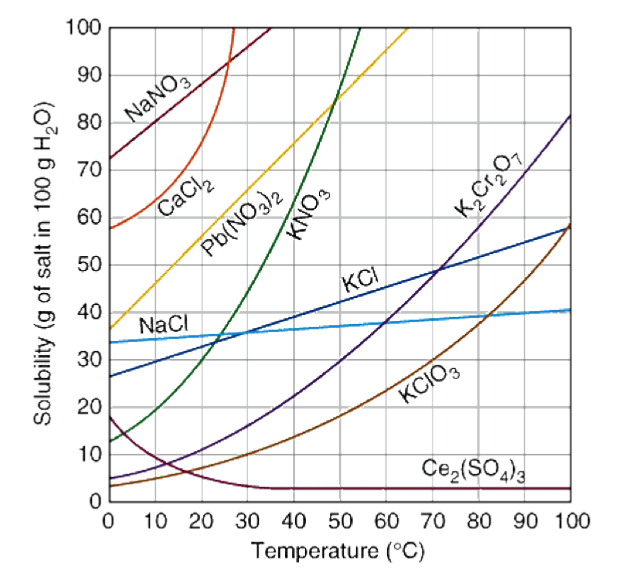
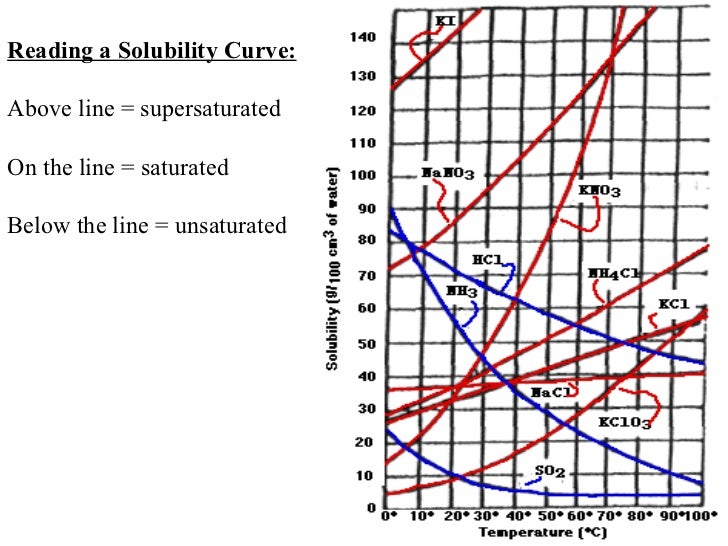
9 types of solution | chemistry. How do we know a solution is supersaturated? Practice reading a solubility graph—part 1.
A supersaturated solution is a solution that contains more than the maximum amount of solute that is capable of being dissolved at a given temperature. Types of saturation. 151k views 2 years ago.
In most cases solubility decreases. Practice reading a solubility graph—part 2. If the solution is unsaturated, the solute will dissolve.
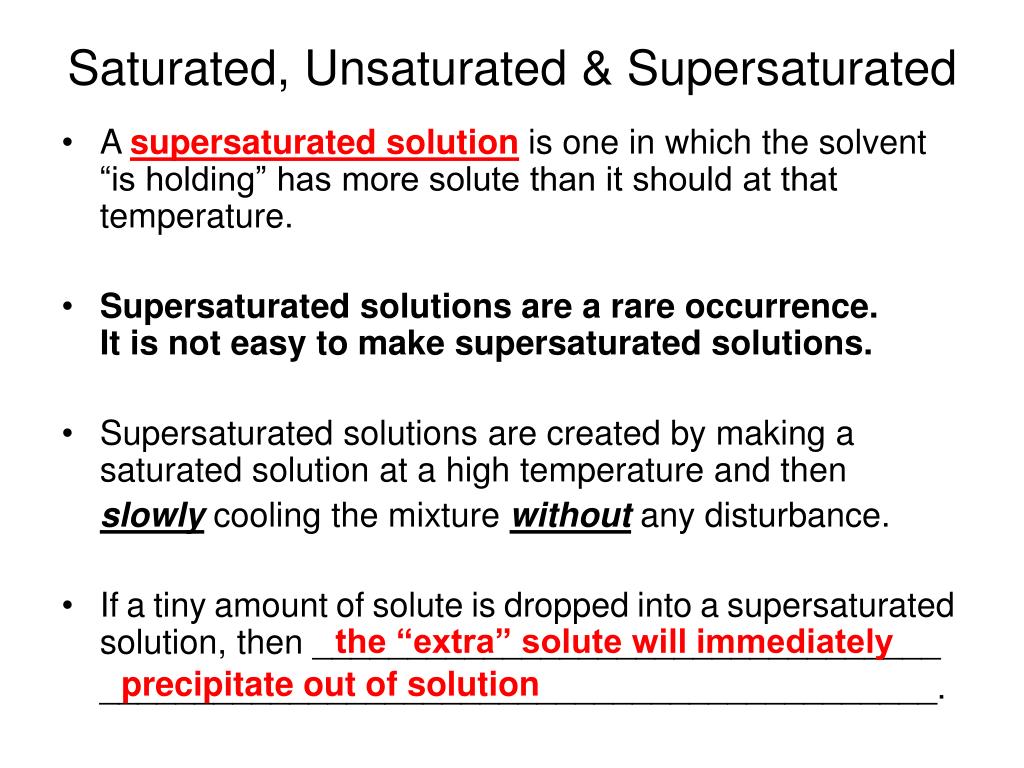
What is a supersaturated solution? A saturated solution is a solution that. It's easy to tell if a solution is unsaturated, saturated, or supersaturated by adding a very small amount of solute.
A solution of this composition is also described as a saturated solution since it can accommodate no more kcl. Under some circumstances it is possible to. In a saturated solution, there is so much solute present that if more were added, it would not dissolve.
A supersaturated solution is a solution, not just technically, but in all sincerity. A supersaturated solution contains more solute at a given temperature than is needed to form a saturated solution. When a seed crystal is.
Solutions may be unsaturated, saturated, or supersaturated, depending on the. How can we cause recrystallization of a supersaturated solution? A supersaturated solution definition is given as the one, which contains more dissolved solute than needed for preparing a saturated solution and is prepared.
The additional solute will not dissolve. In this animated lecture, i will. It is homogenous, clear, transparent, and you'll never tell it from ordinary.
Given scenarios, graphs, diagrams, or illustrations,. A solution of a chemical compound in a liquid will become supersaturated when the temperature of the saturated solution is changed. A supersaturated solution is a solution that contains more dissolved solute than the maximum amount of solute that is capable of being dissolved at a given.